RUG scientists discover how a bacterium ‘eats’
For nine years, Dirk Slotboom has been focused on a single protein. And not just any protein: an ECF transporter, which is essential for getting vitamins outside of a cell into it. And now, the day has finally come: together with research assistants Lotteke Swier and VIDI recipient Albert Guskov, he has figured out how the protein does this. Today, the results will be published in the high-impact Nature Communications.
Molecular motor
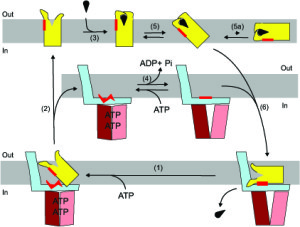
It turns out that the protein functions as a very special motor. That is because the ‘motor’ works fundamentally differently from any other known motors, all of which use a so-called ‘power stroke’ that transforms combustible energy into motion. Slotboom’s protein, however, works like a ‘Brownian ratchet’, a type of motor for which there was no experimental evidence – until now. The motor is set into motion by thermal energy that is already present in the protein due to the random movements of molecules, so-called Brownian motion. After this movement, it is stuck, like a ratchet that can only move forward and not backwards.
‘This is evidence that such a movement really exists’, Slotboom says enthusiastically. ‘People in the field are fascinated!’
Savagely scooped
The importance of this is proven by the fact that over the past few years, Slotboom has been ‘savagely scooped’ no less than three times by Chinese competitors who published elements of the process just before he did – in Nature, of all places. ‘They probably managed to figure it out due to lectures I actually gave’, says Slotboom laconically. ‘But fortunately, everything has turned out all right with this publication.’
The protein in question catches the vitamin by spreading its two ‘arms’, ensnaring the vitamin and then tipping over. ‘That motion happens on its own without any extra energy’, says Slotboom. Next, it attaches itself to another protein that is inside the cell, which leads to the release of the vitamin inside the cell. ‘The proteins are very stable. It’s kind of like a dead end.’
Outstretched arms
In order to escape, the bacterium used ATP, the cell’s loose change, so the protein can tip back up to grab a new vitamin with its outstretched arms.
Then, the motor – or rather the ratchet – has made a full turn and is ready for the next. ‘ATP is only needed for a reset.’
Slotboom’s research is fairly fundamental; no one has ever been able to figure out how this protein worked. But applications for the protein are possible in the future. ‘If you make sure that the protein can’t escape from that dead end by messing with how ATP works, you can block the vitamin transfer. And then you’ve almost got an antibiotic’, says Slotboom.